
Introduction
This patent focuses on a probiotic composition that alleviates hyperuricemia (elevated uric acid levels), a common metabolic disorder. The composition includes two key probiotic strains:
· Akkermansia muciniphila Akk11 (CCTCC NO: M2024119)
· Lactobacillus johnsonii LJ09 (CGMCC NO.20123)
Both strains exhibit synergistic effects that improve uric acid metabolism, enhance kidney clearance, and reduce uric acid synthesis, resulting in kidney protection.
Background
Hyperuricemia can lead to health issues like gout, kidney stones, and renal failure. Current treatments, such as uric acid inhibitors, have limitations, including side effects and resistance. Probiotics, which regulate gut microbiota and influence metabolism, offer potential as alternative therapies for managing uric acid levels. However, probiotic solutions for hyperuricemia are still underexplored, presenting an opportunity for innovation.
Key Components
The patent introduces a probiotic formulation containing Akk11 and LJ09. The key characteristics are:
1. Composition: Akk11 and LJ09 strains are mixed in varying ratios (1:10 to 10:1) to achieve optimal results.
2. Forms of Administration: The probiotic can be delivered in various forms, such as solutions, freeze-dried powders, capsules, tablets, or granules.
3. Dosage: The total viable cell count in the formulation is not less than 1×10^8 CFU/mL or CFU/g.
Mechanisms of Action
The patent highlights the following mechanisms through which the Akk11 and LJ09 alleviate hyperuricemia:
1. Regulation of Uric Acid Metabolism: The combination significantly regulates uric acid production and excretion, reducing serum uric acid levels.
2. Kidney Protection: The composition improves kidney function by reducing uric acid-induced damage and promoting uric acid clearance.
3. Inhibition of Uric Acid Synthesis: It effectively inhibits key enzymes involved in uric acid production, minimizing its formation in the body.
Experimental Results
A series of experiments using mice models demonstrated the efficacy of the probiotic composition. Key findings include:
1. Kidney Function Improvement: Mice treated with the probiotic showed significant improvements in kidney function, evidenced by reduced urine protein and urea nitrogen levels. Additionally, the probiotic increased uric acid excretion, indicating enhanced kidney performance.
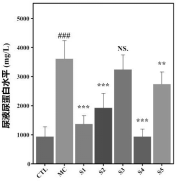
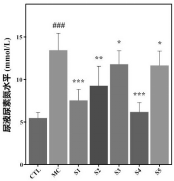
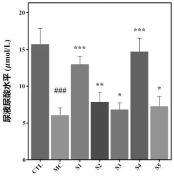
2. Biochemical Marker Analysis: Probiotic-treated mice exhibited reduced serum uric acid, urea nitrogen, and creatinine levels, showing a protective effect on kidney function and efficient waste elimination.
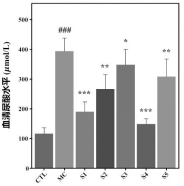
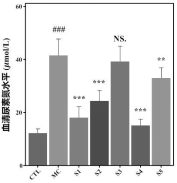
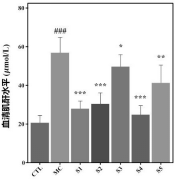
3. Xanthine Oxidase Activity: The composition reduced xanthine oxidase activity in the liver, a key enzyme in uric acid production, further supporting its role in lowering uric acid levels.
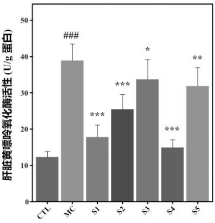
Potential Applications
The probiotic composition is primarily intended for use in preparing pharmaceuticals for preventing or treating hyperuricemia. It may also serve as a health supplement for individuals with elevated uric acid levels. The composition can be enhanced with additives such as fillers, emulsifiers, and prebiotics to improve efficacy and stability.
Conclusion
This probiotic composition, containing Akkermansia muciniphila Akk11 and Lactobacillus johnsonii LJ09, offers a novel and safe approach to managing hyperuricemia. Its synergistic action not only regulates uric acid levels but also protects the kidneys, reducing the risk of complications associated with high uric acid. This invention has potential for use in both pharmaceutical and nutraceutical products aimed at individuals with hyperuricemia or related metabolic disorders.


